The seven-valent pneumococcal conjugate vaccine (PCV7) provided a paradigm shift in the prevention of serious pneumococcal disease in infants. The original vaccine has subsequently been updated with more serotypes added (first bringing the total to 13, then to 20). This is intended to provide broader coverage against invasive disease and combat serotype replacement. However, there are some concerns that serotype-specific protection decreases when valency increased. Immunogenicity is commonly measured with two assays: an ELISA assay that measure geometric mean concentrations of circulation antibody (IgG) or the geometric mean titer of a functional assay that measures activity of those antibodies (opsonophagocytic assay, OPA). To simplify comparisons, we included only clinical trials where a 3+1 schedule was used (three primary doses given in the first year of life, followed by a booster around 1 year of age). First, we’ll examine the seven serotypes that started it all: 4, 6B, 9V, 14, 18C, 19F, and 23F. These seven serotypes are present across all three vaccine generations, PCV7, PCV13, and PCV20. The lines in the plots below show the ‘correlate of protection’, which is a useful, if imperfect, measure used to evaluate the concentration of antibody required to prevent invasive pneumococcal disease in infants.
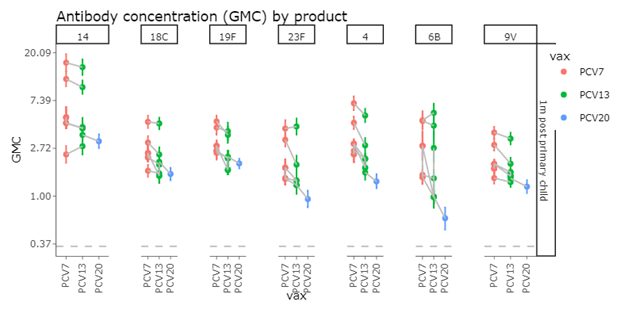
Post Primary IgG GMCs
One month after a three-dose primary series, mean IgG responses to the seven original serotypes decrease as valency increases, with PCV20 producing consistently lower responses than PCV13, after PCV13 produced consistently lower responses than PCV7. Even with the lower antibody responses, the concentrations are above the correlate of protection for preventing invasive pneumococcal disease.
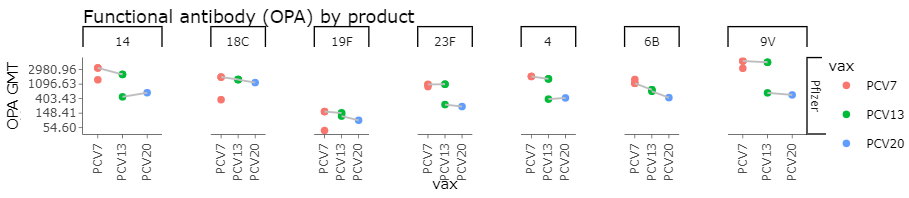
Post Primary OPA GMTs
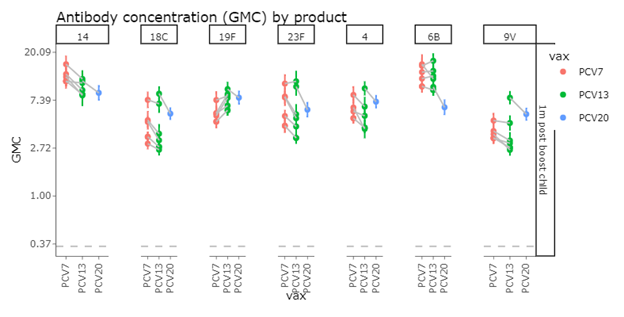
Post Booster IgG GMCs
This pattern continues when re-measured one month after the booster dose.
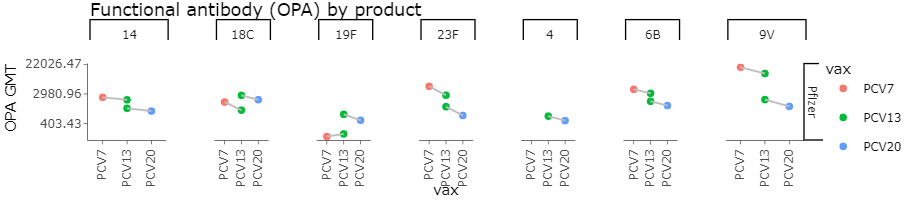
Post Booster OPA GMTs
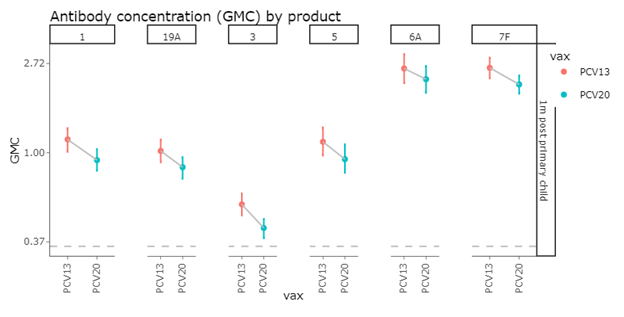
The six additional serotypes present in both PCV13 and PCV20 are 1, 3, 5, 6A, 7F, and 19A. Comparing these serotypes, we see a similar decreasing pattern when examining the shift from IgG GMCs measured in PCV13 clinical trials and those measured in PCV20 clinical trials.
Post Primary IgG GMCs
Following three primary doses, PCV20 elicited diminished IgG responses to the six serotypes added in PCV13.
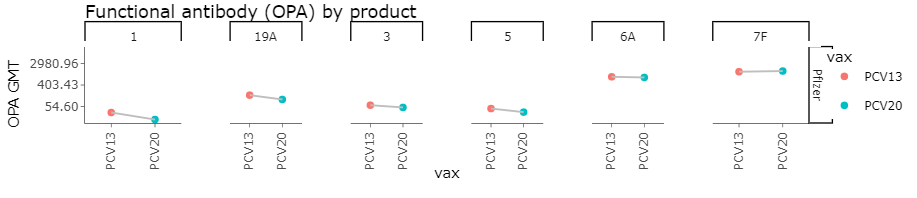
Post Primary OPA GMTs
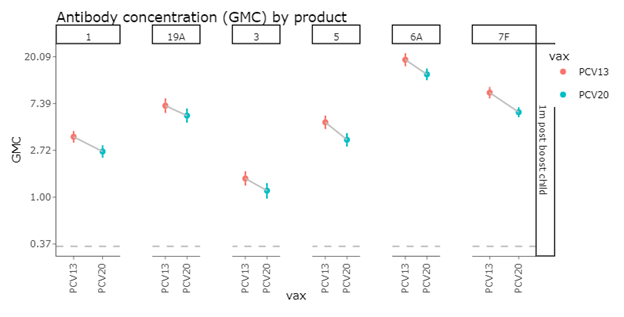
Post Booster IgG GMCs
A similar pattern was seen when examining the measurements taken one month following the booster dose.
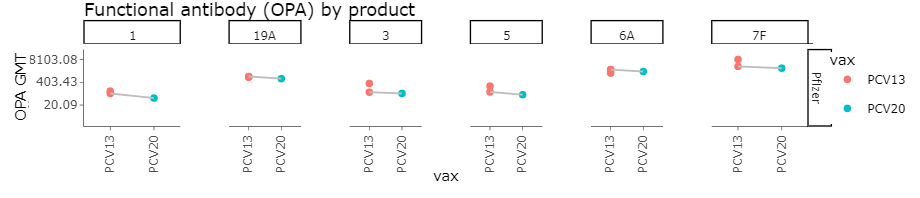
Post Booster OPA GMTs
Now, what does this mean? None of the reductions with increased valency result in lower GMCs than the correlate of protection, typically thought to be between 0.35- 0.40 μg/mL, so it could be that the benefits of increased valency outweigh the risks of diminished protection, but it remains to be seen how this plays out at the population level. The correlate of protection for colonization is likely higher (requiring more antibody to prevent mucosal colonization), so even if we are above the correlate of protection for disease, it is possible we will have reduced ability to reduce circulation of vaccine-targeted serotypes. It should also be noted that even if the protection is lower, the new vaccines protect against more serotypes. Modeling work would need to be done to evaluate the net effect of the vaccine, including diminished immunogenicity and greater serotype coverage.